Recent work by a team of researchers has shown that human brain organoids implanted into the cortex of a mouse brain are both functionally and physically integrated into the mouse host brain. Through the use of transparent graphene microelectrodes and two-photon imaging, researchers were able to show that the implanted human brain organoid responds to light stimuli as well as that the organoid is vascularized by the mouse tissues.
Organoids are small organ-like structures derived from stem cells. In this study, the organoids were generated by taking skin cells and reprogramming them to induce pluripotency, the ability to differentiate into any cell type in the body. These cells are known as induced pluripotent stem cells. iPSCs can then be differentiated into the desired cell type, in this case human brain cells, and grown in 3D culture to create organoids.
There are many potential uses for organoids in science. Organoids can be used to study organ development or to study disease states that affect specific organs. Furthermore, organoids have the potential to one day be used to restore organ function for people whose organs are damaged or no longer functioning.
Despite this potential, organoids have some limitations. Specifically, organoids lack blood vessels which allow for the exchange of nutrients and waste which therefore allow for organ growth. Furthermore, organoids lack many of the immune cells present in our organs. As a result, organoids cannot perform the full function of the organ they are modeling.
The team of researchers led by Duygu Kuzum from the UC San Diego Department of Electrical and Computer Engineering and Anna Devor from the Boston University Department of Biomedical Engineering developed an innovative method to study how organoids integrate into the host upon transplantation.
Researchers removed a small portion of the retrospinal cortex in a mouse brain and implanted a human cortical organoid in its place. In addition to the organoid, researchers also implanted a transparent microelectrode array to monitor the electrical signaling of the organoid and surrounding host brain tissue.
The microelectrodes used in this study were developed by Dr. Kuzum’s lab in 2014. The unique transparency of the graphene microelectrodes allows for collecting both visual and electrical data from a single animal.
Three weeks post implant, the human brain tissue showed signs of responding to an external light stimulus in a similar fashion to the surrounding mouse tissue. This data indicates that the implanted tissue was receiving input from the surrounding mouse tissue enabling it to respond to stimuli.
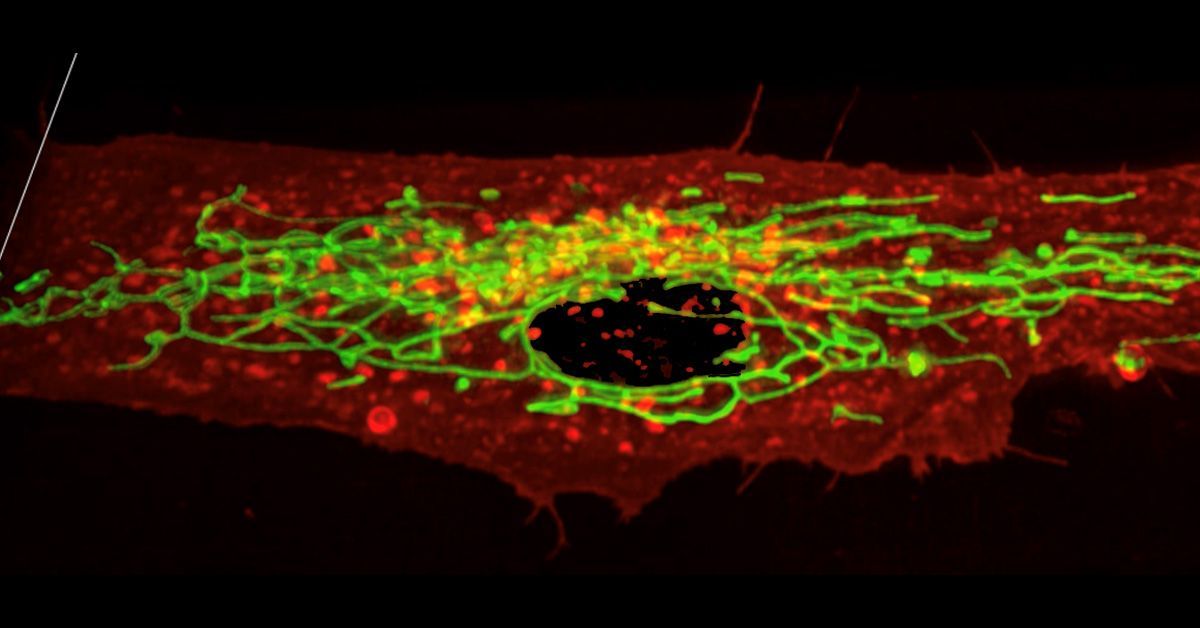
Due to the transparent nature of the microelectrode array, researchers were also able to study vascularization of the human organoid using two-photon microscopy. This microscopy technique allows for imaging thick tissues as well as live tissues. The researchers found that the human cortical organoid transplant was vascularized. While the amount of vascularization was less than that seen in the mouse brain tissue, the organoid was still connected to the host through blood vessels.
One of the lead authors on the paper, Madison Wilson, senior electrical and computer engineering doctorate student at UCSD, noted that the most distinguishing factor of the work is their ability to collect data longitudinally.
“What’s important about the longitudinal study is that we implanted the array and we sealed the craniotomy with a glass window and from then on we didn’t need any additional surgeries,” Wilson said. “So we could record both electrically and optically and visually inspect the area without needing to perturb the mouse any more.”
This study is the first to show functional responses of organoid transplants to external stimuli and also the first to develop a technique to simultaneously study functional and morphological integration of human organoids into mouse models.
“This is the first step in proving that exogenous tissue can be implanted in a brain and gain similar functions to the surrounding cortex,” she said.
The researchers hope that this work will enable the study of disease states in living organisms as well as advance the field toward using organoids to restore damaged or non functioning brain regions.
Additional local collaborators include researchers from Alysson Muotri’s lab at UCSD and Fred Gage’s lab at the Salk Institute.
Art by Ava Bayley for the UCSD Guardian


















Nestor Hernandez • Feb 16, 2023 at 6:30 pm
This is a really incredible discovery! Who would’ve thought that rats were able to accept at least a small part of a human brain! I will share this amazing article with the people from my favorite cleaning services columbus indiana
eva • Jan 11, 2023 at 11:47 am
I honestly don’t see why more people haven’t done this given that I work two shifts, two during the day and two during the evening. And I surely received a $29,000 check. Being able to work from ac57 home allows me to spend more time with my children, which is wonderful.
.
.
See this article for further details—————————————>>> WORK AT HOME