Led by graduate student Michael Schoof at Dr. Peter Walter’s lab in the UC San Francisco Department of Biochemistry and Biophysics, the team engineered antibodies from camelids to immobilize SARS-CoV-2, the virus responsible for COVID-19. Known as “AeroNabs,” these nanobodies provide a promising, immediate therapeutic treatment for COVID-19 illness before vaccines become widely accessible.
“Vaccination is critical, but it may take years before the whole world can get vaccinated,” Michael said. “So throughout that process and even after you still want a therapeutic treatment or post exposure prophylactic to be available.”
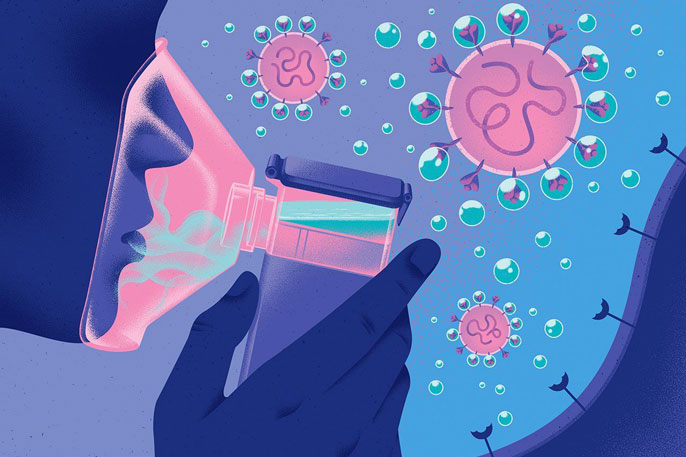
AeroNabs have several advantages over traditional therapeutic antibodies. AeroNabs are made of smaller, more stable camelid-derived antibodies (nanobodies) rather than typical antibodies. Therefore, these molecules are inexpensive to mass produce in bacteria or yeast, and they are stable to transport in the form of powders. Furthermore, AeroNabs can be easily aerosolized and self-administered with a nasal spray or inhaler.
The research team has recently completed testing AeroNabs on hamsters and are working their way to get AeroNabs into the clinics as the world is concentrated on developing vaccines and traditional antibodies. The team is also continuing to develop more versions of AeroNabs to target newly emerged variants of viral SARS-CoV-2.
“As the dynamics of the virus change over the coming year, especially that there’s a new potential pandemic continues to emerge, you can image there’s a version of virus really resistant to vaccine effort and there’s a new version of vaccine that only provides 50 percent effectiveness, I think options like this [nanobody treatment] become far more important [in this situation],” said Dr. Aashish Manglik, the co-principal investigator of the project.
The virus SARS-CoV-2 attacks a target lung cell by latching its spike protein to fit perfectly onto the human protein receptors, ACE2, which cover the surface of the cell. It is only after this interaction that the virus is able to enter the human cell and forcibly direct it to spread the viral RNA, causing a cascade of cell infection. However, with the AeroNabs developed by the UCSF team, this chance is greatly reduced because the virus binding with ACE2 is blocked by its binding to these nanobodies — an immune agent designed with a greater affinity to SARS-CoV-2.
“Antibody” is a general name for proteins that defend against foreign objects such as pathogenic bacteria and viruses in a host animal. Unlike the traditional antibodies found in mammals, these single-domain antibodies (nanobodies) originated from camelids, such as llama and alpacas, and are missing the light chain of a typical antibody, resulting in a simpler and more stable structure to engineer. Recently developed nanobody technology took advantage of these structural properties to expand nanobody applications in medicine and therapeutics.
The original discovery of nanobody by Belgian biology professor Raymond Hamers in the late 1980s was unexpected. After finding a sample of carmel serum in the lab, Hammers assigned his undergraduate students to work on isolating antibodies from the sample. After purification, they discovered undocumented mini-antibodies. Further characterization revealed that these mini-antibodies belonged to a new class which they named “nanobodies”. Since Nature published this finding in 1993, nanobody technology has made notable progress in its application. For example, the FDA approved its use in treating a rare blood clotting disorder in 2019. This new biotechnology attracted many labs to employ it as a tool to develop therapeutics. Among them is Manglik’s lab at UCSF.
Manglik spent the past three years building the world’s largest library of nanobody DNA sequences with Dr. Andrew Kruse at Harvard Medical School. The library houses over two billion nanobody-expressing yeast cells. This powerful resource not only saves the time-consuming labor of harvesting nanobodies from the blood of llamas or camels, it’s also shared with the world to give hundreds of labs access to studying nanobodies.
“My lab normally studies something completely different, we are very interested in the receptors that enable our bodies to see, smell and taste,” Manglik said. “In order to study these receptors, we developed in the past these little antibodies to push and pull and manipulate these receptors to function.”
Walter and Manglik already teamed up before their project on tackling coronavirus with nanobodies. As a fourth year graduate student in the Walter lab, Michael specialized in studying the regulation of a protein named “eIF2B,” which is essential for protein translation and appears to be a critical regulatory hub during traumatic brain injury and other neurological disorders. Walter saw the potential application of nanobodies in modulating the behavior of eIF2B, and the partnership was soon made.
When COVID-19 surged through America in early 2020 and most non-COVID related research was closed down, the Waler lab promptly switched its gears to research SARS-CoV-2 with Manglik for a solution: Can nanobodies effectively halt the spread of the virus?
“Michael really took the lead on using some of the expertise he gained from my lab to find nanobodies against the spike protein,” Manglik said regarding their collaborative project on COVID-19.
The team screened through Manglik’s yeast surface-display library to narrow down the population that would likely bind to SARS-CoV-2 spikes. Within three weeks, they identified 800 potential candidates. Michael mixed these individual yeast cultures with fluorescently labeled SARS-CoV-2 Spike and excess human ACE2 protein. The preliminary result showed many nanobody expressedexpressing yeast, but the 21 top candidates showed competition with the ACE2 protein. These nanobodies were later classified into two types. Class 1 nanobodies, namely Nb6 and Nb11, competed directly with ACE2 for the same binding site on the spike, and Class 2 nanobodies such as Nb3 targeted a different domain. As both classes revealed decreased binding activity in the presence of increased ACE2, this classification would allow the team to identify the most potent candidate and engineer an ultrapotent version of itself.
The task was split among researchers, including biochemists and virologists across the campus and globe. The team collaborated with the QCRG Structural Biology Consortium to image the interaction between Nb6 and SARS-CoV-2 Spike using atomic-resolution cryo-electron microscopy. This data provided critical information about how Nb6 blocks ACE2 binding and allowed the team to further optimize the molecule into Nb6-tri by linking three Nb6 nanobodies with flexible linkers of amino acids — the basic structural unit of a protein.
“We have made the nanobodies look more like a human antibody through a humanization process,” Michael said. “It’s purely interacting with the viral protein and unlikely to have side effects.”
To examine its efficacy, Manglik connected with former UCSF postdoc Marco Vignuzzi to test Nb6-tri against live virus at his Biosafety Level 3 (BSL-3) laboratory located at Institut Pasteur in Paris. The result proved that this three-armed nanobody binds exceptionally well to SARS-CoV-2, making it a promising antiviral.
“Our thought is not to unnecessarily compete with the vaccine already deployed right now,” Manglik said. “It’s really to think about where an approach like this [nanobody] can have some utility.”
Months of work condensed into a paper published in Science last December. More than 50 researchers co-authored to propose the effective treatment of using this synthetic nanobody to inactivate SARS-CoV-2.
Photo courtesy of Kouzou Sakai for Folio Art.














Gayle B Ashton • May 3, 2021 at 12:23 pm
I am anxious for this to come on the market. My son-in-law is a lung transplant patient, he received the Pfizer vaccine but it didn’t produce antibodies. Doubtless, there are other immune-suppressed patients for whom this is a life-saving solution.
Jenny Molloy ARNP • Mar 31, 2021 at 1:28 pm
I’ve been following this amazing discovery since summer 2020 and am trying to wait patiently for this incredible nanobody to hit the market. This is a true game changer in the world of anti-virals. I strongly support an EUA ASAP!! This seems to be a front runner for getting us back to business and life! With all thats happening in our world we really really need something positive.
Regards
Emma • Mar 26, 2021 at 10:35 am
Hi, thank you very much for your articles. I think that research in the health sector is very important. I recently read about the Herbalife Ingredients Overview – https://www.techtimes.com/articles/252373/20200908/herbalife-ingredients-and-botanical-innovation.htm which will help you better understand the quality of supplements.
Dan Fries • Mar 9, 2021 at 5:57 pm
Great work! And nice to hear some news, I’ve been waiting since August.
This quote was telling:
“Our thought is not to unnecessarily compete with the vaccine already deployed right now,” Manglik said. “It’s really to think about where an approach like this [nanobody] can have some utility.”
hmmm… wonder why:
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/emergency-use-authorization-medical-products-and-related-authorities?fbclid=IwAR2EDZHh4gOkHZi6BKpR99aTzsyE6KjyP33tzChFVutWvm-B2KqbpTas2V0#euas
“For FDA to issue an EUA, there must be no adequate, approved, and available alternative to the candidate product for diagnosing, preventing, or treating the disease or condition. A potential alternative product may be considered “unavailable” if there are insufficient supplies of the approved alternative to fully meet the emergency need. A potential alternative product may be considered “inadequate” if, for example, there are contraindicating data for special circumstances or populations (e.g., children, immunocompromised individuals, or individuals with a drug allergy), if a dosage form of an approved product is inappropriate for use in a special population (e.g., a tablet for individuals who cannot swallow pills), or if the agent is or may be resistant to approved and available alternative products.”
Emma • Mar 7, 2021 at 3:37 am
Drawing conclusions, it can be noted that raising the immunity of an adult at home is quite a feasible task, I advise you to learn more about it here https://zestywell.com/glutathione-and-immunity/ . It is important for a strong immune system-to lead a proper lifestyle and meet the new day with a good mood.
Nathan Lind • Mar 1, 2021 at 7:13 am
Two things. First, this is amazing research! Can I get on the list to be notified when this is available?
Second, the advertisement at the top of this webpage, changes every second or two, with each advertisement being a slightly different height. This causes the text of the article to move up and down every second or two. Frustratingly hard to read on mobile.